Dr. Robert Hsu from the University of Southern California, Norris Comprehensive Cancer Center, describes a recent research paper he co-authored that was published by Oncotarget, entitled, “Molecular characterization of Kita-Kyushu lung cancer antigen (KK-LC-1) expressing carcinomas.”
Behind the Study is a series of transcribed videos of researchers elaborating on their oncology-focused research published by Oncotarget. Visit the Oncotarget YouTube channel for more insights from outstanding authors.
—
Hi, my name is Robert Hsu. I’m a medical oncologist, affiliated with the University of Southern California, Norris Comprehensive Cancer Center.
I wanted to introduce our paper titled, “Molecular characterization of Kita-Kyushu lung cancer antigen (KK-LC-1) expressing carcinomas,” in which we studied a cancer testis antigen called KK-LC-1, also known as CT 83, which has been noted to be expressed in multiple solid tumors. Most notably lung, gastric, and breast cancer. As there are multiple new clinical trials out there looking at adoptive cell therapy targeting this cancer testis antigen, we wanted to see which non-small cell lung cancer patients in a clinical trial might be a best fit for this. Working with Caris Life Sciences, we looked at 9,790 non-small cell tumors that underwent whole transcriptom sequencing. We split these tumors into quartiles based on KK-LC-1 expression and investigated pathological and molecular differences.
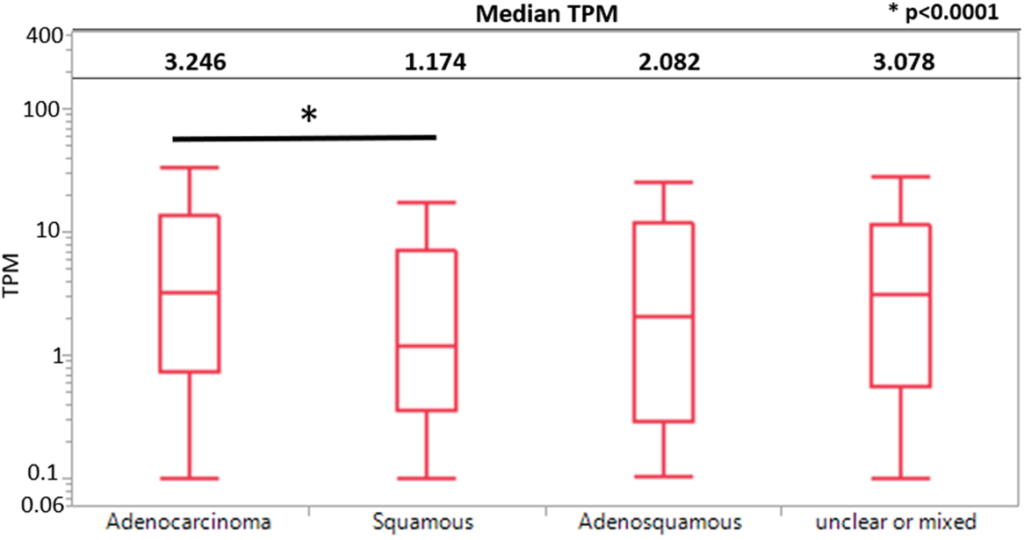
We saw that adenocarcinoma had significantly higher KK-LC-1 expression than squamous cell carcinoma. We saw that tumors with the highest quartile of KK-LC-1 expression had a greater proportion of tumors with higher tumor mutational burden greater than 10. 44% in the highest quartile of KK-LC-1 expression compared to 28% in the lowest quartile of KK-LC-1 expression. We did not see any differences in the percentage of PD-L1 positive expression across all four quartiles. We saw that increased KK-LC-1 expression was associated with increased M1 macrophage abundance. We saw that higher levels of KK-LC-1 expression were seen in pan-wild type and KRAS mutated tumors and associated with high tumor mutational burden.
From this, we showed that many of the tumors had high KK-LC-1 expression were those without actual mutations, except for KRAS G12C. And interestingly, we did not see a difference in PD-L1 positivity across all four quartiles of KK-LC-1 expression. These findings make us believe that adoptive cell therapy targeting this cancer testis antigen should be considered as therapy.
I wanted to take on this research as I found this to be an opportunity to collaborate with some of the leading thought leaders in thoracic oncology, along with the researchers at University of Southern California, who have been looking at preclinical studies for possible T-cell receptor therapy. What I found most notable in our work was that the tumors that had high KK-LC-1 expression were primarily those without actual mutations, except for those with KRAS G12C. And interestingly, at the same time, not seeing a difference in PD-L1 positivity across all four quartiles. This work needs to be evaluated at protein expression level, but really made paved a way to how we evaluate patients more suitable to adoptive cell therapy in non-small cell lung cancer in the future.
I also really enjoyed getting a chance to present these findings with members of the Caris Precision Oncology Alliance team, or some of the leading thought leaders in the field. Since publication of this manuscript, we have worked with pathology at University of Southern California to create a panel including KK-LC-1 that can be validated at a protein expression level. We had a small sample size of 23 patients with known KK-LC-1 protein expression and analyzed the seven-plex multi immunofluorescent panel looking at protein expression of different immune infiltrates in these patients. We showed a significantly increased density of CD8 T-cells and tumor cells compared to stromal cells in tumors with the highest gene expression of KK-LC-1. We also saw an increased cell density of regulatory T-cells and tumor cells in tumors with the highest gene expression of KK-LC-1.
These findings were suggestive of increased immune infiltrates and tumor cells and in tumor cells relative to stromal cells. And that strategy is to deplete regulatory T-cells and the further amplify CD8 T-cells should be pursued in the development of T-cell receptor therapy targeting KK-LC-1 in the future. In the meantime, we have continued to collaborate with the basic science lab at University of Southern California to help with development of a drug targeting KK-LC-1.
I’d like to thank all the authors in this study in helping with this collaboration. In particular, Dr. Nieva, for his mentorship and guidance with creation of this manuscript. I’d also like to thank Caris Life Sciences for their work in providing us with this notable data and support.
My experience publishing with Oncotarget was a very positive experience. I appreciated the time in which they were able to find the reviewers to consider our manuscript, and clear communication with us and letting us know about the status of our journal. I felt that the process working with the processing team to help create the proofs was very prompt and straightforward. I was impressed with how quickly the proofs were made and how professional the paper looked once produced.
Click here to read the full study published by Oncotarget.
WATCH: Lung cancer playlist on Youtube
READ: Special collection on lung cancer
ONCOTARGET VIDEOS: YouTube | LabTube | Oncotarget.com
—
Oncotarget is a unique platform designed to house scientific studies in a journal format that is available for anyone to read—without a paywall making access more difficult. This means information that has the potential to benefit our societies from the inside out can be shared with friends, neighbors, colleagues and other researchers, far and wide.
For media inquiries, please contact media@impactjournals.com.