Behind the Study is a series of transcribed videos from researchers elaborating on their oncology-focused studies published by Oncotarget. A new Behind the Study is released each Monday. Visit the Oncotarget YouTube channel for more insights from outstanding authors.
—
Dr. Tapasree Roy Sarkar from the Department of Biology at Texas A&M University describes a research paper she co-authored in 2021 that was published by Oncotarget, entitled, “Carcinoma cells that have undergone an epithelial-mesenchymal transition differentiate into endothelial cells and contribute to tumor growth.”
Dr. Tapasree Roy Sarkar
Hi, I am Dr. Tapasree Roy Sarkar. I am a faculty in the Department of Biology at Texas A&M University. Thank you so much for giving me a chance today to talk about my paper that was recently published at Oncotarget. The title of the paper is, “Carcinoma cells that have undergone an epithelial-mesenchymal transition differentiate into endothelial cells and contribute to tumor growth.”
First, I would like to say thank you to all my authors — we have graduate students, undergraduate students, post-doctoral scientist — and the faculties from Texas A&M University, University of Texas MD Anderson Cancer Center, and also from Hamamatsu University School of Medicine in Japan. So they worked really hard to publish this paper.
Now, let me begin with the history of the research leading up to this paper. So we know that angiogenesis is a normal physiological process that entails the development of new blood vessels to remodeling of pre-existing vasculature that underpin by endothelial cell sprouting, proliferation, and fusion. That ability of solid tumor to induce and sustain angiogenesis is termed as neoangiogenesis, especially in the cancer context, and it has been recognized as one of the distinguishing hallmarks of cancer.
Accordingly, the induction of this angiogenesis that helps the tumor grow beyond a certain critical size. That means when the preexisting tissue’s vasculature becomes inadequate to support the ever-increasing growth of this tumor demand, then this angiogenesis happens. In addition to ensuring the availability of essential nutrients and oxygen, the establishment of new blood vessels also facilitates the removal of cytotoxic metabolic byproducts and carbon dioxide and, crucially – the most important thing – this angiogenesis process also helps the shed tumor cells to disseminate. They travel through those blood vessels, go to distant locations, and they form secondary tumor or metastasis.
Now, as the rapidly developing tumors, they outgrow their blood supply. They begin to exhibit areas of localized oxygen deprivation, and this is known as hypoxia. This hypoxia, initially it induces EMT, which is known as epithelial to mesenchymal transition, that allows the cells to change their properties from epithelial and become more invasive and aggressive mesenchymal-like cells. This hypoxia, in turn, it tilts the balance towards the pro-angiogenic factor and then activating the angiogenic switch, and it’s helping this tumor or hypoxia induced neoangiogenesis process.
At the molecular level, an important nexus in tumor progression is the activation of this angiogenic switch that govern the balance between multitude of pro and anti-angiogenic factor that regulate the endothelial cell proliferation and migration. So there are different ways this neoangiogenesis can happen.
I’m going to talk about four most important ones.
So the dominance of pro-angiogenic factor that promote the growth of new blood vessels from the pre-existing vessels through the so-called sprouting angiogenesis process. In addition to that, there is another process where the bone marrow-derived endothelial progenitor cells that can be mobilized to initiate the de-novo cell formation in response to angiogenic signal, and that is known as vasculogenesis. However, there are some tumors that can be avascular tumor. They can nevertheless grow, at least initially, and without evoking any angiogenic response, through the process that is known as vessel co-option. And what happens during that time is in this non-angiogenic mode, which mostly prevails in highly vascularized host-tissues, the tumor cells hijack the host vasculature and they migrate along with the pre-existing blood vessels that’s invading the surrounding tissues. The fourth option, which is known as vascular mimicry, that entails the de-novo generation of microvessels that are lined with very highly aggressive tumor cells, embedded in a rich extracellular matrix, and they are mimicking the true vascular endothelium-like structure, but they are lacking the endothelial cell markers, such as CD31 or CD34. Therefore, the complex mechanism underlying this neoangiogenesis defer from this physiological angiogenesis and lead to the formation of dysfunctional and disorganized blood vessels with a defective endothelial layer. Nevertheless, that fuels tumor progression and helping the tumor to grow.
As I just mentioned that, in addition to this angiogenesis, hypoxia is also inducing epithelial to mesenchymal transition. Epithelial to mesenchymal transition isn’t an (inaudible) embryonic problem that is misappropriated during this carcinoma progression, and it is a complex series of cellular reprogramming event that facilitates the conversion of immotile, polarized epithelial cells into more intrinsically migrate or invasive and aggressive mesenchymal counterparts. We previously identified that one transcription factor, Foxc2. During my post-doctoral study at MD Anderson, I work with this Foxc2 transcription factor, and it is a key downstream effector of several converging EMT pathways that functions to confer the mesenchymal and stem cell traits underpinning the metastatic competence. Also interestingly, Foxc2 has been linked to angiogenesis, both in the context of normal development and tumor progression. It has the ability to transcriptionally regulate different pro-angiogenic factors such as vascular endothelial growth factor, platelet-derived growth factor, angiopoietin-2.
We also reported that the epithelial cells that induce to undergo EMT, they exhibit multi-lineage differentiation potential, similar to mesenchymal stem cells. So they display the ability to differentiate into major mesodermal lineages, such as osteoblast, adipocyte, and chondrocyte.
So given those old facts together, we then decided to see, or we sought ascertain, with the cells that have undergone EMT in the hypoxic milieu, can accrue these endothelial cell attributes and therefore, augments tumor growth by directly contributing to the tumor vasculature. As I just mentioned, all those different procedure of endothelial … angiogenesis process, as we know that also they are trying to mobilize, the endothelial progenitor cells are coming on the blood vessels. Here, we are trying to see that (inaudible), which induce this EMT procedure.
So in this study, what we are trying to say is that the cancer cells that undergone EMT, they can trans-differentiate into endothelial cells and they can form the blood vessels, helping in this angiogenesis process. So they are not depending on the endothelial progenitor cells that can come from outside and form the blood vessels, they can be independent. They can make their own blood vessels. So our study showed direct evidence that the tumors can be independent.
Next, I’m going to talk about the most notable part of my work. So we have done a lot of in-vitro studies, as well as in-vivo studies. So in-vitro studies, we have used triple-negative breast cancer cell lines, which has EMT-induced cell lines. We have used epithelial cells, which we use as a control, and we have also HMLE-Snail, HMLE-Twist cell lines, which are also EMT-induced cell lines. For in-vitro studies, we try to culture those cells in the endothelial media and we try to see that this incubation with the endothelial media, they are trans-differentiating into endothelial cells and showing endothelial cell-like property.
For example, we try to see the expression pattern of CD31, which is a very commonly known endothelial marker. And if we observed that those EMT-induced cells, when we incubated them with the endothelial media, they were expressing high CD31. We have performed the tube-like formation of capillary-like structure, which is also characteristics of endothelial cells. Indeed, we have seen that the formation of tube-like structure increased when we cultured those cells in endothelial media. We have also performed LDL-uptake assay, we tried to see the internalization of LDL, or low density lipoprotein, the major carrier of cholesterol in the blood, to the receptor-mediated endocytosis. And we observed that these endothelial media cultured mesenchymal cells, they exhibited significant uptake of those fluorescently-tagged LDL. So that is showing that altogether, when we cultured those EMT-induced cells with endothelial media, they are trans-differentiating into endothelial cells.
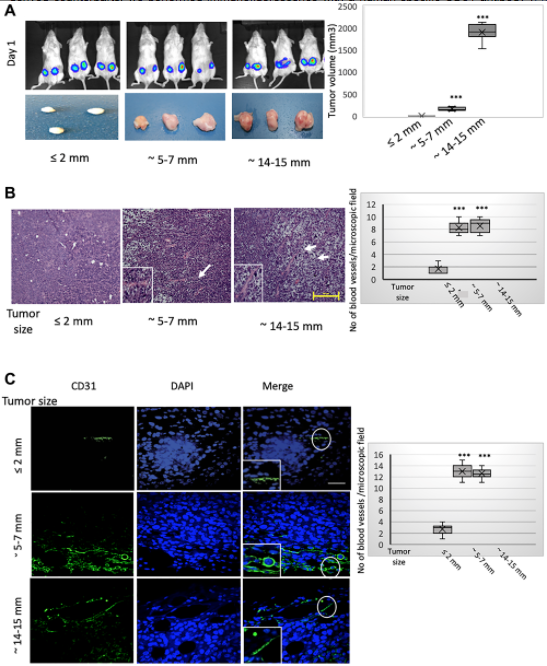
Next, we have performed in-vivo studies and we have done two different types of tumor models. In those tumor models, we used MCF7 cells, and we are growing the tumor a different size. The beginning, as I said, that when the tumor is gradually growing, at the core there should be hypoxia, and we try to see during that hypoxia, HIF-1 alpha find expression, and the HIF-1 alpha expression, or hypoxia, induces EMT. So that expression of EMT markers, along with Foxc2 expression, increased during that time. Once EMT happens, then that helps that trans-differentiation process, so we showed that this neoangiogenesis at the core region of these outgrowth tumors is enacted predominantly through these MCF7 cell trans-differentiation towards an endothelial phenotype, and we showed how these EMT cells is trans-differentiating into endothelial cells.
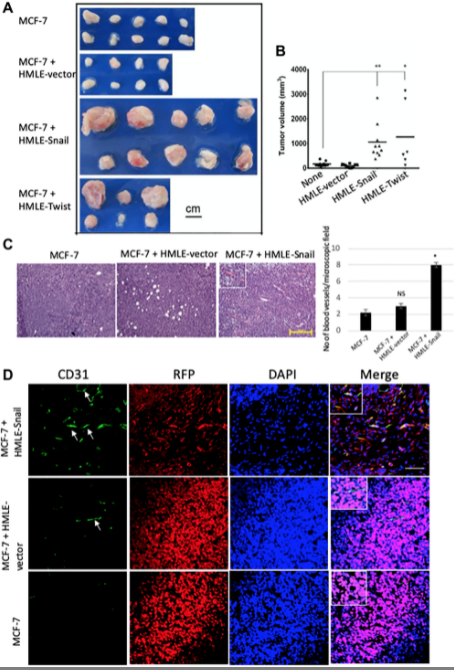
In our second mouse model, what we did is we used MCF7 with the HMLE-Snail, or HMLE-Twist cells, and also we had control cells. So what we did is we also tried to develop the tumor and we showed that this study reinforced that assertion that the capacity to potentiate that endothelial trans-differentiation is predominantly associated with the mesenchymal phenotype of the cells input in this model. So both of those models helped us to understand that how this EMT is helping the trans-differentiation process, EMT-induced cell is trans-differentiating into endothelial cells for the neoangiogenesis process.
We try to see that molecular mechanism, and we observed that the Foxc2, as I mentioned, which is one of the most important factor for the EMT process, and this Foxc2 is playing a central role in regulating the endothelial trans-differentiation of cells that have undergone EMT. We did in-vitro studies and also in-vivo studies with control and Foxc2-silenced cell lines. And we have these beautiful experiments which showed clearly that it is playing a very important role for regulating these endothelial trans-differentiation.
All together in this present study, we demonstrated that the cells that have undergone EMT can promote tumor growth and neovascularization through this acquisition of endothelial-like phenotype, with the central EMT mediator, Foxc2, that is playing a key role in this process. So this finding specifically link the stemness conferred to EMT, to the acquisition of endothelial cell traits and the augmentation of tumor angiogenesis in a Foxc2-dependent manner.
So now what currently we are doing and what is our future plan is, as I said, we started doing the molecular mechanism. We showed the role of Foxc2, but we want to study more. We want to know details about what is going on, how this EMT is helping this trans-differentiation process. So we try to find out the role of different factors, for example, HIF-1 alpha and Foxc2, whether they’re interacting directly. What is the role of … our preliminary study showed the role of Gr1 MDSC — that is myeloid-derived suppressor cells — are also playing some role, and VEGFR-2 or KDR, they are also having some role. So we are trying to find out the interaction between them and their role in this EMT-mediated trans-differentiation, endothelial trans-differentiation process.
We are also doing atomistic using in-vitro as well as in-vivo tumor models. And one particular signaling pathway we are looking also is deap signaling pathway. We’re trying to see whether it has any role in this EMT-mediated trans-differentiation.
At the beginning, when I said that there is another one very interesting way to have this neoangiogenesis process or these blood vessels in the tumor, which is known as vascular mimicry, where this aggressive breast cancer cell lines, you can see that they are forming these blood vessel-like structure, which doesn’t have endothelial-like traits or they don’t express CD31 or CD34. But I want to see whether the EMT is playing any role in this vasculogenic mimicry. Is there any connection between this EMT and this vascular mimicry process? So we are currently doing that one as well.
So at the end of what I want to say, I want to thank so many people. The authors, all the researchers, they worked really hard, and we have done this beautiful study where first time we are showing direct evidence that cancer cells, when they’re inside the tumor, when the tumor cells are growing at the core of the tumor, they are experiencing hypoxia, and once the hypoxia is there, the hypoxia induces EMT. These EMT cells, they can trans-differentiate directly into endothelial cells and they can form their blood vessels. They’re making the tumors independent. They don’t need any endothelial cells or pre-endothelial cells from outside to make blood vessels, they can make their own blood vessels, making the tumor more independent.
So I am really happy about this study and the outcome of this study. I think it’s an amazing study, and I want to thank many people. All the researchers here who helped doing all those amazing experiment. I want to thank Dr. Sendurai Mani, who at MD Anderson Cancer Center was my previous postdoctoral mentor. He helped a lot with many reagents and also with many amazing sedations. I also want to thank my department, Department of Biology at Texas A&M University for all the other help they have provided me to finish up that paper. So thank you so much again, Oncotarget, for giving me a chance to talk about my research, to explain what we did. Thank you so much.
Click here to read the full study published by Oncotarget.
YOU MAY ALSO LIKE: More Oncotarget Videos on LabTube
—
Oncotarget is a unique platform designed to house scientific studies in a journal format that is available for anyone to read—without a paywall making access more difficult. This means information that has the potential to benefit our societies from the inside out can be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact media@impactjournals.com.