Dr. Nerymar Ortiz-Otero from the Meinig School of Biomedical Engineering, Cornell University, discusses a research paper she co-authored that was published by Oncotarget in Volume 11, Issue 12, entitled, “Cancer associated fibroblasts confer shear resistance to circulating tumor cells during prostate cancer metastatic progression.”
Behind the Study is a series of transcribed videos from researchers elaborating on their oncology-focused studies published by Oncotarget. Visit the Oncotarget YouTube channel for more insights from outstanding authors.
—
Good morning. My name is Nerymar Ortiz-Otero. I’m an author for the recent published paper in Oncotarget, “Cancer associated fibroblasts confer shear resistance to circulating tumor cells during prostate cancer metastatic progression.” Today, I’m going to give a little information about this study and what other findings we had that allowed us to go to these kinds of research study.
To give a little information about myself, I was born and raised in Puerto Rico. I did my bachelor degree in University of Puerto Rico at Mayaguez campus. After I graduated with my bachelor degree, I applied to a PhD program at Cornell University in biomedical engineering. My PhD degree is about the analysis and targeting of circulating cells in metastatic cancer patients.
Cancer is the second leading cause of death worldwide, where metastasis is responsible about 90% of these cancer-related deaths. Cancer metastasis is a process where the tumor cells migrate from the primary location to form a secondary tumor in distant organs. During this process, the tumor cells have to detach from the primary tumor and invades the tumor stroma, and then travels into the blood vasculature. When the tumor cells are in the blood vasculature, these are known as circulating tumor cells, and these will travel in the bloodstream until these find a permissive environment to grow and form secondary tumor.
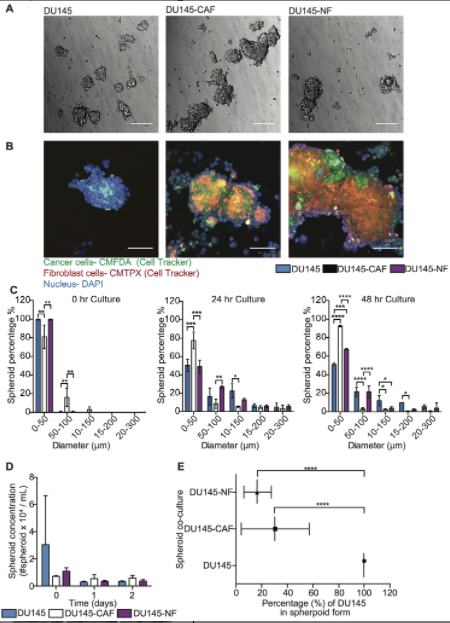
These circulating tumor cells may travel as single CTCs or as CTC aggregates, which is composed by tumor cells, but also by stromal cells as, for example, cancer associated fibroblasts. Cancer associated fibroblasts, known as CAFs, is one of the main component of the tumor microenvironment. It can compose up to 80% percent of the solid tumor. These kind of cells can promote cancer migration, can promote cancer pervasion by several mechanisms. For example, it can promote tumor cells proliferation, it can promote the resistance to cell death, or it can promote cancer invasion and migration. The function of CAFs have been well established by the literature in the primary and in the secondary tumor, however, it still is not clear whether CAF assists in the CTC transit leading to the worsening clinical outcome in cancer patients.
During my PhD degree, I had the chance to isolate and analyze circulating tumor cells and cancer associated fibroblasts from blood samples collected from metastatic cancer patients. In this project, we found that high level of cancer associated fibroblasts correlate with the worsening clinical outcome in this cohort of patients, suggesting the potential use of this as a cancer prognosis biomarker in blood sample collected from cancer patients. Based on these kind of results and the strong correlation that we observed between the level of cancer associated fibroblasts and the worsening clinical outcome in these metastatic cancer patients, we decided to look a little bit closer about the function of these CAFs in the bloodstream. What is the role of CAFs in the bloodstream in cancer metastasis? That’s the main reason why we decided to initiate this study that we’ve recently published in Oncotarget.
In this study, we demonstrated that when the CAFs are interacting with tumor cells and cells aggregates has CTC aggregates that can be released by primary tumors in patients. These kind of aggregates, basically CAFs promote the tumor cells’ survival when these are experiencing high fluid shear stress. Also, CAFs basically in this aggregate form, protect the proliferation capabilities of these tumor cells when these are experiencing high fluid shear stress, which promote cancer pervasion by facilitating the colonization of this in organs.
So here in this paper, we found one other role of these in the bloodstream, which is facilitating the survival of tumor cells in the bloodstream so these can see and form secondary tumors in distant organs. This kind of study explored the function of these CAFs in the bloodstream, but in a specific prostate cancer disease. However, we do now we believe that this kind of behavior could happen in another types of carcinoma.
Now, looking to future direction, we would like to explore a little bit more about the role of CAFs in the bloodstream in other carcinoma, and how we can develop a therapeutic approach to target these CAF population in the bloodstream. If CAFs are necessary to form metastases … If CAFs are one of the cells that promote the tumor cell survival in the bloodstream … If we target this kind of population in the bloodstream, we may have a chance to prevent cancer metastasis. So in the future direction, we would like to explore a little bit more about how we can develop this therapeutic approach to target this CAF population and eliminate these in the bloodstream.
Thank you for listening. I would like to thank all my funding sources, as NSF has this long fellowship that they were really crucial part to complete my PhD. I also would like to thank my advisor, Dr. Michael King, he has been an amazing advisor during this whole process to shape me into the scientist that I am right now. I also would like to thank all the team lab members, the people that I work with in the research lab, because it’s really collaborative environment to work with and I think every student was part of my progress and part of my work. I also would like to thank my company advisor, Dr. David Putnam and Dr. Tracy Stokol, because they were also a crucial part of my PhD dissertation and my PhD studies. And finally, I would like to thank the Diversity Program at Cornell University that has been an amazing support group for diverse student at the University. I’m really thankful for all the advice, all the support that I have received from them since day one. Thank you.
Click here to read the full research paper, published by Oncotarget.
ONCOTARGET VIDEOS: YouTube | LabTube | Oncotarget.com
—
Oncotarget is a unique platform designed to house scientific studies in a journal format that is available for anyone to read without a paywall making access more difficult. This means information that has the potential to benefit our societies from the inside out can be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact media@impactjournals.com.